Translate this page into:
Differentiation of inflammatory papulosquamous skin diseases based on skin biophysical and ultrasonographic properties: A decision tree model
2 Department of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
3 Electrical Engineering Department, Sharif University of Technology, Tehran, Iran
4 Department of Bioelectronic and Biomedical Engineering, School of Advanced Technologies in Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
5 Center for Research and Training in Skin Diseases and Leprosy; Clinical Trial Center, Tehran University of Medical Sciences, Tehran, Iran
Correspondence Address:
Alireza Firooz
Center for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences, 415 Taleghani Avenue, Tehran
Iran
| How to cite this article: Yazdanparast T, Yazdani K, Ahmad Nasrollahi S, Nazari M, Darooei R, Firooz A. Differentiation of inflammatory papulosquamous skin diseases based on skin biophysical and ultrasonographic properties: A decision tree model. Indian J Dermatol Venereol Leprol 2020;86:752 |
Abstract
Background: The biophysical and ultrasonographic properties of the skin change in papulosquamous diseases.
Aims: To identify biophysical and ultrasonographic properties for the differentiation of five main groups of papulosquamous skin diseases.
Methods: Fifteen biophysical and ultrasonographic parameters were measured by multiprobe adapter system and high-frequency ultrasonography in active lesions and normal control skin in patients with chronic eczema, psoriasis, lichen planus, pityriasis rosea and parapsoriasis/mycosis fungoides. Using histological diagnosis as a gold standard, a decision tree analysis was performed based on the mean percentage changes of these parameters [(lesion–control/control) ×100] for differentiation of the diseases.
Results: The accuracy of the decision tree model for differentiation of five diseases was 67% which developed based on changes in stratum corneum hydration, epidermal thickness, skin pH, melanin index, R0 (reciprocal of firmness) and erythema. Among the flowcharts for pairs of diseases, three models for differentiation had high accuracy (> 95%): those of psoriasis from lichen planus, pityriasis rosea, and parapsoriasis/mycosis fungoides.
Limitations: Validation studies on a larger sample size in situations where the diagnosis is unclear are needed to confirm the accuracy and applicability of decision trees.
Conclusion: Skin biophysical and ultrasonographic properties may help in the differentiation of papulosquamous diseases as simple and non-invasive tools.
Introduction
Papulosquamous disorders share a common clinical picture, presenting with scaly papules and plaques while having different etiopathogenesis. The most common papulosquamous disorders are dermatitis, psoriasis, lichen planus, pityriasis rosea and parapsoriasis/mycosis fungoides. Although they are usually differentiated by clinical history and examination, the similarities in their clinical manifestations can generate difficulties in their diagnoses.[1] Histopathologic examination stands as the gold standard for diagnosis and can help in differentiating these conditions; however, it is an invasive procedure.[2]
Various simple, non-invasive methods can make clinical diagnosis more certain.[3] Among them, dermoscopy has been shown to help in the diagnosis of papulosquamous disorders;[4],[5] reflectance confocal microscopy (RCM) can help in differential diagnosis, prognosis and response to treatment of some inflammatory skin conditions and avoids biopsies;[3],[6],[7] optical coherence tomography is a promising non-invasive method for diagnosis of inflammatory skin diseases,[8],[9],[10],[11] and an algorithmic method for pattern analysis of these diseases has been proposed.[12]
Nowadays, novel and non-invasivein vivo methods for measurement of skin biophysical and biomechanical properties such as stratum corneum hydration, transepidermal water loss, friction and elasticity are available for assessing skin lesions. These can be used to increase the diagnostic accuracy, thereby avoiding a biopsy although their exact diagnostic application is not clear yet.[13]
High-frequency ultrasonography is another non-invasive method that can be used to evaluate response to treatment and disease progression in papulosquamous diseases.[14],[15],[16],[17] Although ultrasonography is an established diagnostic tool in dermatology,[16],[18] it is not commonly used in the differentiation of papulosquamous diseases and only some changes such as epidermal thickening and subepidermal low echogenic band have been shown by ultrasound in inflammatory diseases.[17],[19]
Decision tree—a type of algorithm which simplifies the decision making in the presence of uncertainty—may be useful in conjunction with these non-invasive methods. The tree starts with node, main decision and the lines extending out from this node for each possible solution. If the solution leads to another decision, the new line extends to the next possible series of choices which overall provides a supportive decision-making process.[20]
Ultrasonography in combination with skin biometry has been used to evaluate some types of papulosquamous disorders previously; however, we were unable to find any comprehensive study to compare the biophysical, biomechanical and sonographic characteristics of these diseases to reach a practical diagnostic approach. The purpose of this study was to make a decision tree for diagnosis of papulosquamous disorders using biophysical, biomechanical and ultrasonographic properties of skin.[21],[22]
Methods
All patients suffering from inflammatory skin diseases including chronic eczema, psoriasis, lichen planus, pityriasis rosea and parapsoriasis/mycosis fungoides stage 0/1 who had been referred to the Center for Research and Training in Skin Diseases and Leprosy from September 2014 to March 2016 and fulfilled eligibility criteria were enrolled.
The criteria for enrolment were the following: mild to moderate inflammatory skin diseases based on the dermatologist's diagnosis (AF), minimum age 18 years, nonpregnant and not lactating, having active lesions with a maximum duration of 4 weeks at the moment of examination, skin types 3 or 4 in Fitzpatrick classification, receiving no treatment for index lesion, no history of any other skin diseases within the 3 months prior to the enrollment, having no systemic diseases that might affect the skin[23]
This study protocol was approved by the ethics committee of Tehran University of Medical Sciences and was conducted according to the Declaration of Helsinki; ethical considerations such as providing oral informed consent and confidentiality were made. All the measurements were non-invasive and done free of charge.
The clinical diagnosis was confirmed with histological findings as the gold standard.
The participants were asked not to wash their skin or use any topical products from the night prior to the measurements. To perform the biophysical measurement, patients were requested to rest and relax for 20 minutes in the standard atmosphere conditions (20-25° C temperature; 25-30% humidity). Then the stratum corneum hydration (using Corneometer® CM 825), transepidermal water loss (using Tewameter® TM 300), pH (using Skin-pH-Meter® PH 905), erythema and melanin index (using Mexameter® MX 18), sebum (using Sebumeter® SM 815), friction value (using Frictiometer FR700), skin temperature (using Skin-Thermometer ST 500) and elasticity parameters including R0, R2 and R5 (using Cutometer® 580) were measured by Multi Probe Adapter (MPA, Courage + Khazaka electronic GmbH, Germany).
R0 is a passive behavior of the skin to force; it shows firmness or stretchability of the skin and represents total elastic and plastic deformation of the skin. R2 shows gross viscoelasticity in percent, it represents resistance to the mechanical force versus the ability to return. R5 demonstrates net elasticity or elastic portion of the suction part versus the elastic portion of the relaxation part in percent.[24]
In addition to the parameters mentioned above, the thickness and echo-density of dermis and epidermis were measured by high-frequency ultrasonography device (DUB skin scanner, tpm Company, Luneburg, Germany, using 22 MHz and 50 MHz probes of DUB skin scanner respectively), on the day of measurement.
All measurements were performed on one selected active lesion, one uninvolved perilesional area defined as normal-appearing skin about 3 cm away from the border of active disease and one uninvolved symmetrical area defined as normal-appearing skin on the same location on the opposite side of the body.
The biophysical tests were done by a person who was unaware of the clinical and pathological diagnosis of the patient.
Descriptive statistics measures, including frequency, mean and standard deviation were used to describe the data including baseline characteristics and biophysical and ultrasonographic parameters.
To eliminate the impact of different anatomical site of measurements, the mean percentage changes of parameters were calculated according to the below formula in any diseases:
([lesion parameter – control parameter]/control parameter) × 100
Decision tree analysis was performed for differential diagnosis of the five diseases and also for each pair of diseases based on the mean percentage changes of any biophysical and ultrasonographic parameters. The control parameter mentioned in the formula was the mean of perilesional and symmetrical skin parameters because there were no statistically significant differences between them by paired T-test in all five diseases.
We used Matlab software (2016, UK) to design a decision tree. By default, fitctree function used the standard Classification and Regression Tree algorithm to create decision trees.[25],[26] K-fold cross-validation method has been used to validate the algorithm. This method is a powerful methodology to prevent data over-fitting.[27] In this study, the fold value was set to K=10.
Results
One hundred and eleven (111) participants (56 female and 55 males) were included and had the following diagnosis: 22 chronic eczema, 26 psoriasis, 21 lichen planus, 21 pityriasis rosea and 21 parapsoriasis/mycosis fungoides. The mean age of participants was 38.97 (SD = 14.47) years. The mean duration of the disease was 4.74 (SD = 7.54) years. The index lesions were located on upper limbs in 54 patients, lower limbs in 23 patients, trunk in 29 patients and face in 5 patients. The time interval between histological diagnosis and biophysical assessment was maximum of 3 days and no clinical intervention was performed during this period.
The best decision tree model for differential diagnosis of all five diseases based on changes in stratum corneum hydration, epidermal thickness, pH, melanin index, R0 (reciprocal of skin firmness), and erythema had an overall accuracy of 67% to differentiate all of the five diseases [Figure - 1].
 |
| Figure 1: The flowchart of decision tree to differentiate 5 inflammatory skin diseases based on skin biophysical properties, the accuracy of generated decision tree was 67% (TPR: True positive rate) |
The sensitivity and confusion matrix of the decision tree are shown in [Table - 1]. The diameter of the table is the same as the sensitivity concept; the rest of the houses of each row are false negative. The most possible confusion was related to pityriasis rosea which could be differentiated from parapsoriasis/mycosis fungoides with a 47.5% chance.
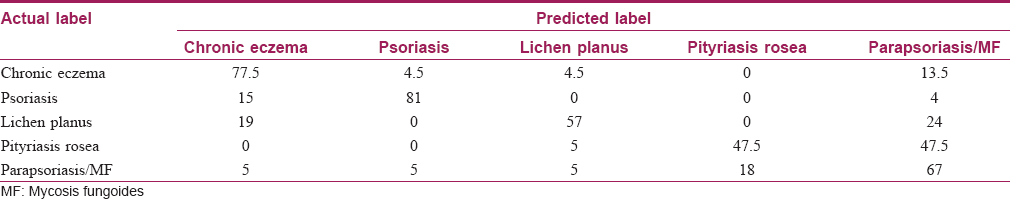
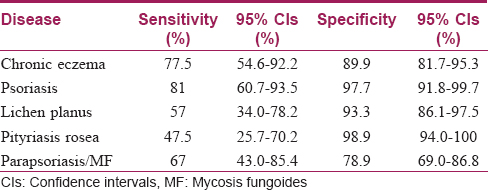
The sensitivity, specificity and 95% confidence intervals of this diagnostic decision tree for these five papulosquamous diseases are given in [Table - 2].
[Figure - 2] shows the best decision tree models for pairwise differentiation of five diseases based on skin biophysical and ultrasonographic properties.
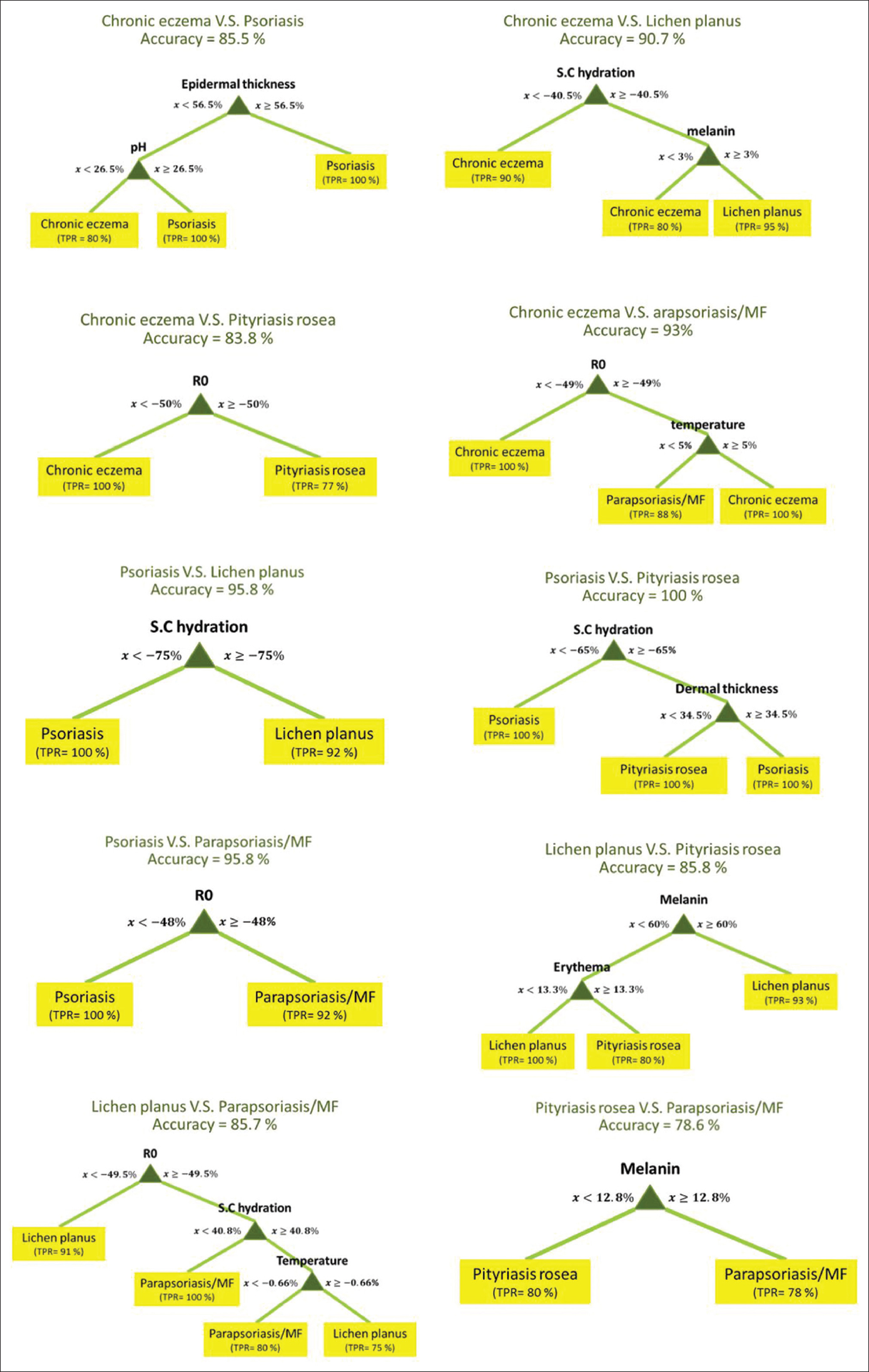 |
| Figure 2: The flowcharts of decision tree models for pairwise differentiation of inflammatory skin diseases based on skin biophysical properties |
Among these flowcharts, psoriasis vs. pityriasis rosea flowchart had the highest (100%) and pityriasis rosea vs parapsoriasis/mycosis fungoides flowchart had the lowest (78.6%) accuracy.
Discussion
There is an overlap of clinical features of papulosquamous disorders, which often makes clinical diagnosis difficult. There is an overlapping of some histological features as well although some features might be characteristic for each disease.[28]
This study showed that certain biophysical and ultrasonographic properties of the skin can be used as a noninvasive approach in the differentiation of five inflammatory papulosquamous diseases: chronic eczema, psoriasis, parapsoriasis/mycosis fungoides, lichen planus and pityriasis rosea.
The change in R0 provided the highest diagnostic yield in differentiating all five diseases. However, the differentiation ability improved when skin parameters were combined and gave an accuracy of 67%. Hydration of stratum corneum was the first predictive parameter followed by the epidermal thickness and skin pH which helped to differentiate psoriasis from chronic eczema. On the other hand, higher melanin content was in favor of lichen planus, whereas R0 and erythema values helped to differentiate chronic eczema, parapsoriasis/mycosis fungoides and pityriasis rosea.
As stratum corneum hydration is an important marker of the epidermal barrier function, it is reasonable to be considered as the first step of the differentiation criteria. Lower stratum corneum hydration compared to the unaffected adjacent and symmetric skin area was in favor of diagnosis of chronic eczema and psoriasis.[29] This is in agreement with previous studies in which the decrease of stratum corneum hydration in dermatitis and psoriasis were reported.[30],[31]
The next differentiating feature between chronic eczema and psoriasis was epidermal thickness which was significantly thicker in psoriasis. This is consistent with histological findings in which psoriasis has markedly more epidermal hyperproliferation than atopic dermatitis.[32]
An increase in skin pH has been reported in both eczema and psoriasis lesions.[33],[34],[35] We found that psoriatic skin is more alkaline than chronic eczema. High pH is frequently associated with high transepidermal water loss which itself is associated with low hydration.[36] The higher skin pH in psoriatic lesions in comparison to chronic eczema lesions could be attributed to less stratum corneum hydration in psoriatic lesions because of more epidermal hyperproliferation.[32]
The other branch of decision tree distinguishes lichen planus from other diseases by the amount of melanin increase. We observed that more than 60% increase in skin melanin content is in favor of lichen planus. Post-inflammatory hyperpigmentation due to damage to basal layer cells is a characteristic clinical feature of lichen planus, and melanin increase is a diagnostic feature for lichen planus.[37]
The next distinguishing characteristic in the decision tree is R0 (reciprocal of firmness), and a decrease of more than 50.2% was in favor of the diagnosis of chronic eczema. This finding is justifiable as superficial firmness of the skin mainly correlates with the stratum corneum hydration[38] and this decreases significantly in dermatitis lesions.[39] Moreover, fibrosis of the papillary dermis was reported to be one of the best features of chronic dermatitis using an ensemble of classifiers for the diagnosis of erythemato-squamous diseases.[40] This finding is in accordance with this branch of the decision tree that shows more skin firmness to be in favor of chronic eczema given that fibrosis increases skin firmness.
In the last branch of the decision tree, the amount of increase in skin erythema was decisive to differentiate parapsoriasis/mycosis fungoides from pityriasis rosea. Clinically, the lesions of pityriasis rosea tend to be more erythematous, while the erythema in parapsoriasis/mycosis fungoides is faint. Red blood cell extravasation is a histological feature of pityriasis rosea.[41]
Based on the results of the confusion matrix, the most possible difficulty was found when trying to differentiate between pityriasis rosea and parapsoriasis/mycosis fungoides as many of the histological features also overlap.[33]
In our decision tree model, there was no confusion between chronic eczema and pityriasis rosea, psoriasis and pityriasis rosea, lichen planus and pityriasis rosea, psoriasis and lichen planus. Non-invasive measurement of skin properties may be particularly useful in the differentiation of these diseases.
Among the decision tree models for differentiation of each pair of diseases, some had more than 95% accuracy. The amount of stratum corneum hydration decrease was useful to differentiate psoriasis and lichen planus with more than a 75% decrease favoring psoriasis. The relationship between the decrease in stratum corneum hydration and psoriasis has been shown previously.[31] Since the epidermal layer acts as a barrier to keep skin homeostasis, impairment of its function leads to loss of skin hydration.[42]
In addition to stratum corneum hydration, the amount of dermal thickness increase was useful to differentiate psoriasis and pityriasis rosea. If the dermal thickness increase was more than 34.5%, the diagnosis of psoriasis was proposed. Thickening of the dermis is one of the main sonographic features in psoriasis. This thickness is a result of an abnormal concentration of pro-inflammatory cells and a hypoechoic band in the upper dermis, which may represent edema and vasodilatation within the papillary dermis.[43]
An increase of more than 48% in lesion stiffness could differentiate psoriasis from parapsoriasis/mycosis fungoides. Stiffness increase or dispensability decrease was proven in psoriatic plaques previously and it was negatively correlated with psoriasis severity index.[44]
Overall, this study showed that biophysical properties have high accuracy in differentiating psoriasis from lichen planus, pityriasis rosea and parapsoriasis/mycosis fungoides with practical application.
The differentiation of papulosquamous diseases using voting feature intervals based on clinical and histopathological features of diseases such as erythema, scaling, itching, melanin incontinence and fibrosis of the papillary dermis has been shown previously.[45] Also, adaptive neuro-fuzzy inference system or combined neural networks model have been used for differential diagnosis of papulosquamous diseases based on clinical and histopathological features.[37],[46] But the difficulty is that a disease may show overlapping histopathological features with another disease, especially at the early stages.[45]
The present study is one of the few studies to assess biophysical parameters for the differentiation of papulosquamous diseases.
However, this study has some limitations. First, we used several parameters to create decision trees, but only some of them were useful and applicable in the algorithm. Future studies can be performed based on only the applicable parameters derived from this study. Low number of cases is another limitation. Further validation studies on a larger number of patients in situ ations where the diagnosis is unclear are needed to confirm the accuracy of these decision trees and their applicability to the method in real-life scenarios.
Using a neural network model of skin, biophysical properties have relatively acceptable predication accuracy (67%) for the diagnosis of papulosquamous diseases. The skin biophysical and ultrasonographic properties can serve as practical and useful references in the diagnosis of papulosquamous diseases to minimize unnecessary procedures and improve care quality.
With the increasing use of new technologies such as skin biometrology and ultrasonography, our decision tree flowcharts may help dermatologists to differentiate papulosquamous diseases in a non-invasive and real-time approach.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal the identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study was supported by a research grant from CRTSDL (grant number: 94-01-34-28430) and two research awards were provided by the Ministry of Health and Medical Education of Iran and Lierac Company.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Norman RA, Blanco PM. Papulosquamous diseases in the elderly. Dermatol Ther 2003;16:231-42.
[Google Scholar]
|
| 2. |
Younas M, Haque A. Spectrum of histopathological features in non-infectious erythematous and papulosquamous diseases. Int J Pathol 2004;2:24-30.
[Google Scholar]
|
| 3. |
Calzavara-Pinton P, Longo C, Venturini M, Sala R, Pellacani G. Reflectance confocal microscopy forin vivo skin imaging. Photochem Photobiol 2008;84:1421-30.
[Google Scholar]
|
| 4. |
Alinda MD, Hutomo M, Setyaningrum T. Dermoscopy supports the diagnose of papulosquamous disorders. Period Dermatol Venereol 2014;26:1-7.
[Google Scholar]
|
| 5. |
Lallas A, Kyrgidis A, Tzellos TG, Apalla Z, Karakyriou E, Karatolias A, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br J Dermatol 2012;166:1198-205.
[Google Scholar]
|
| 6. |
Ardigo M, Longo C, Gonzalez S, International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from the International Confocal Working Group: Specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol 2016;175:364-74.
[Google Scholar]
|
| 7. |
Astner S, Gonzalez E, Cheung A, Rius-Diaz F, González S. Pilot study on the sensitivity and specificity ofin vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol 2005;53:986-92.
[Google Scholar]
|
| 8. |
Morsy H, Kamp S, Thrane L, Behrendt N, Saunder B, Zayan H, et al. Optical coherence tomography imaging of psoriasis vulgaris: correlation with histology and disease severity. Arch Dermatol Res 2010;302:105-11.
[Google Scholar]
|
| 9. |
Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: Technical and clinical aspects. Arch Dermatol Res 2011;303:457-73.
[Google Scholar]
|
| 10. |
Welzel J, Bruhns M, Wolff HH. Optical coherence tomography in contact dermatitis and psoriasis. Arch Dermatol Res 2003;295:50-5.
[Google Scholar]
|
| 11. |
Gambichler T, Pljakic A, Schmitz L. Recent advances in clinical application of optical coherence tomography of human skin. Clin Cosmet Investig Dermatol 2015;8:345-54.
[Google Scholar]
|
| 12. |
Boone M, Norrenberg S, Jemec G, Del Marmol V. High-definition optical coherence tomography: Adapted algorithmic method for pattern analysis of inflammatory skin diseases: A pilot study. Arch Dermatol Res 2013;305:283-97.
[Google Scholar]
|
| 13. |
Cal K, Zakowiecki D, Stefanowska J. Advanced tools forin vivo skin analysis. Int J Dermatol 2010;49:492-9.
[Google Scholar]
|
| 14. |
Dasgeb B, Kainerstorfer J, Mehregan D, Van Vreede A, Gandjbakhche A. An introduction to primary skin imaging. Int J Dermatol 2013;52:1319-30.
[Google Scholar]
|
| 15. |
Polańska A, Silny W, Jenerowicz D, Knioła K, Molińska-Glura M, Dańczak-Pazdrowska A. Monitoring of therapy in atopic dermatitis–observations with the use of high-frequency ultrasonography. Skin Res Technol 2015;21:35-40.
[Google Scholar]
|
| 16. |
El-Zawahry MB, Abdel El-Hameed El-Cheweikh HM, Abd-El-Rahman Ramadan S, Ahmed Bassiouny D, Mohamed Fawzy M. Ultrasound biomicroscopy in the diagnosis of skin diseases. Eur J Dermatol 2007;17:469-75.
[Google Scholar]
|
| 17. |
Polanska A, Danczak-Pazdrowska A, Silny W, Sadowska A, Jenerowicz D, Osmola-Mankowska A, et al. High-frequency ultrasonography in monitoring the effects of treatment of selected dermatoses. Postepy Dermatol Alergol 2011;28:255-60.
[Google Scholar]
|
| 18. |
Ulrich J, Schwürzer-Voit M, Jenderka KV, Voit C. Sonographic diagnostics in dermatology. J Dtsch Dermatol Ges 2014;12:1083-98.
[Google Scholar]
|
| 19. |
Wortsman X, Wortsman J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J Am Acad Dermatol 2010;62:247-56.
[Google Scholar]
|
| 20. |
Podgorelec V, Kokol P, Stiglic B, Rozman I. Decision trees: An overview and their use in medicine. J Med Syst 2002;26:445-63.
[Google Scholar]
|
| 21. |
Polańska A, Dańczak-Pazdrowska A, Silny W, Jenerowicz D, Olek-Hrab K, Osmola-Mańkowska A. Nonlesional skin in atopic dermatitis is seemingly healthy skin-observations using noninvasive methods. Wideochir Inne Tech Maloinwazyjne 2013;8:192-9.
[Google Scholar]
|
| 22. |
Holm EA, Wulf HC, Thomassen L, Jemec GB. Instrumental assessment of atopic eczema: Validation of transepidermal water loss, stratum corneum hydration, erythema, scaling, and edema. J Am Acad Dermatol 2006;55:772-80.
[Google Scholar]
|
| 23. |
Lamb RC. Skin manifestations of systemic disease. Medicine 2017;45:399-404.
[Google Scholar]
|
| 24. |
Neto P, Ferreira M, Bahia F, Costa P. Improvement of the methods for skin mechanical properties evaluation through correlation between different techniques and factor analysis. Skin Res Technol 2013;19:405-16.
[Google Scholar]
|
| 25. |
Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression trees. Boca Raton: Chapman & Hall; 1993.
[Google Scholar]
|
| 26. |
Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: Methodological review and comparison with logistic regression. Ann Behav Med 2003;26:172-81.
[Google Scholar]
|
| 27. |
Kothari R, Dong M. Decision trees for classification: A review and some new results. In: Pal SK, Pal A, editors, editors. Pattern Recognition from Classical to Modern Approches. Singapore: World Scientifi; 2000. p. 169-84.
[Google Scholar]
|
| 28. |
Chichani S, Negi SR, Kalla AR, Gaur S. Study of histopathology of papulosquamous lesion of skin a prospective and retrospective study. Int J Appl Res 2016;2:115-7.
[Google Scholar]
|
| 29. |
Darlenski R, Sassning S, Tsankov N, Fluhr JW. Non-invasivein vivo methods for investigation of the skin barrier physical properties. Eur J Pharm Biopharm 2009;72:295-303.
[Google Scholar]
|
| 30. |
De Marchi F, Piacentini GL, Piazza M, Sandri M, Boner AL, Peroni DG. Correlation of skin barrier impairment in atopic dermatitis with aeroallergen sensitization. Allergy Asthma Proc 2015;36:e127-33.
[Google Scholar]
|
| 31. |
Lee Y, Je YJ, Lee SS, Li ZJ, Choi DK, Kwon YB, et al. Changes in transepidermal water loss and skin hydration according to expression of aquaporin-3 in psoriasis. Ann Dermatol 2012;24:168-74.
[Google Scholar]
|
| 32. |
Bovenschen HJ, Seyger MM, Van de Kerkhof PC. Plaque psoriasis vs. atopic dermatitis and lichen planus: A comparison for lesional T-cell subsets, epidermal proliferation and differentiation. Br J Dermatol 2005;153:72-8.
[Google Scholar]
|
| 33. |
Xie H, Li L, Xiong LD, Liao F, Zhang GR. The changes of skin barrier of patients with different facial dermatitis and the comparison of CE and KLK5. Sichuan Da Xue Xue Bao Yi Xue Ban 2013;44:940-4, 998.
[Google Scholar]
|
| 34. |
Shi VY, Leo M, Hassoun L, Chahal DS, Maibach HI, Sivamani RK. Role of sebaceous glands in inflammatory dermatoses. J Am Acad Dermatol 2015;73:856-63.
[Google Scholar]
|
| 35. |
Ye L, Lv C, Man G, Song S, Elias PM, Man MQ. Abnormal epidermal barrier recovery in uninvolved skin supports the notion of an epidermal pathogenesis of psoriasis. J Invest Dermatol 2014;134:2843-6.
[Google Scholar]
|
| 36. |
Choi SJ, Song MG, Sung WT, Lee DY, Lee JH, Lee ES, et al. Comparison of transepidermal water loss, capacitance and pH values in the skin between intrinsic and extrinsic atopic dermatitis patients. J Korean Med Sci 2003;18:93-6.
[Google Scholar]
|
| 37. |
Ubeyli ED, Güler I. Automatic detection of erthemato-squamous diseases using adaptive neuro- fuzzy inference systems. Comput Biol Med 2005;35:421-33.
[Google Scholar]
|
| 38. |
Sasai S, Zhen YX, Suetake T, Tanita Y, Omata S, Tagami H. Palpation of the skin with a robot finger: An attempt to measure skin stiffness with a probe loaded with a newly developed tactile vibration sensor and displacement sensor. Skin Res Technol 1999;5:237-46.
[Google Scholar]
|
| 39. |
Sugarman JL, Fluhr JW, Fowler AJ, Bruckner T, Diepgen TL, Williams ML. The objective severity assessment of atopic dermatitis score: An objective measure using permeability barrier function and stratum corneum hydration with computer-assisted estimates for extent of disease. Arch Dermatol 2003;139:1417-22.
[Google Scholar]
|
| 40. |
Nanni L. An ensemble of classifiers for the diagnosis of erythemato-squamous diseases. Neurocomputing 2006;69:842-5.
[Google Scholar]
|
| 41. |
Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases 2017;5:203-11.
[Google Scholar]
|
| 42. |
Lee HJ, Lee SH. Epidermal permeability barrier defects and barrier repair therapy in atopic dermatitis. Allergy Asthma Immunol Res 2014;6:276-87.
[Google Scholar]
|
| 43. |
Gutierrez M, Wortsman X, Filippucci E, De Angelis R, Filosa G, Grassi W. High-frequency sonography in the evaluation of psoriasis: nail and skin involvement. J Ultrasound Med 2009;28:1569-74.
[Google Scholar]
|
| 44. |
Choi JW, Kwon SH, Youn JI, Youn SW. Objective measurements of erythema, elasticity and scale could overcome the inter- and intra-observer variations of subjective evaluations for psoriasis severity. Eur J Dermatol 2013;23:224-9.
[Google Scholar]
|
| 45. |
Güvenir HA, Demiröz G, Ilter N. Learning differential diagnosis of erythemato-squamous diseases using voting feature intervals. Artif Intell Med 1998;13:147-65.
[Google Scholar]
|
| 46. |
Übeyli ED. Combined neural networks for diagnosis of erythemato-squamous diseases. Expert Syst Appl 2009;1:5107-12.
[Google Scholar]
|
Fulltext Views
6,598
PDF downloads
3,361





